You must login before you can run this tool.
Multicellular modeling of SARS-CoV-2 dynamics and virion mediated cell fusion with antiviral drug effects
Simulate SARS-CoV-2 dynamics and virion mediated cell fusion with antiviral drug effects using PhysiCell
Category
Published on
Abstract
Virion pharmacodynamics and cell fusion tissue simulator
This model simulates viral dynamics of SARS-CoV-2 (coronavirus / COVID19) in a layer of epithelium and pharmacodynamics response. It is being rapidly prototyped and refined with community support (see below).
This multiscale simulator combines several model components, including Tissue, ACE2 receptor dynamics, Viral replication, Single-cell response and Pharmacodynamics submodel. We also modified the epithelium dynamics submodel to simulate Cell fusion process. Please see PhysiCell model for COVID19 for whole model information.
In the pharmacodynamics submodel, we model the maximum multiplication response vector rmultiplication as virion response to drug dose. We assume drug has a Hill coefficient (n) and half-max effect drug concentration (EC50). We use a Hill effects model:
E = Emax cn / ( EC50n + cn).
For each cell's current effect E, we set its multiplication rate to:
rmultiplication = d0 + (dmax − d0) E / Emax S
In the above equation, d0 is background rates; dmax is drug induced maximum damage to multiplication rates; S is the drug efficiency. We also need to mention that we calculate three kinds of drug effects in the model, extracellular and intracellular as well as cell fusion, for modeling inhibiting endocytosis, virion replication, and cell fusion processs. Please see the diagram as following:
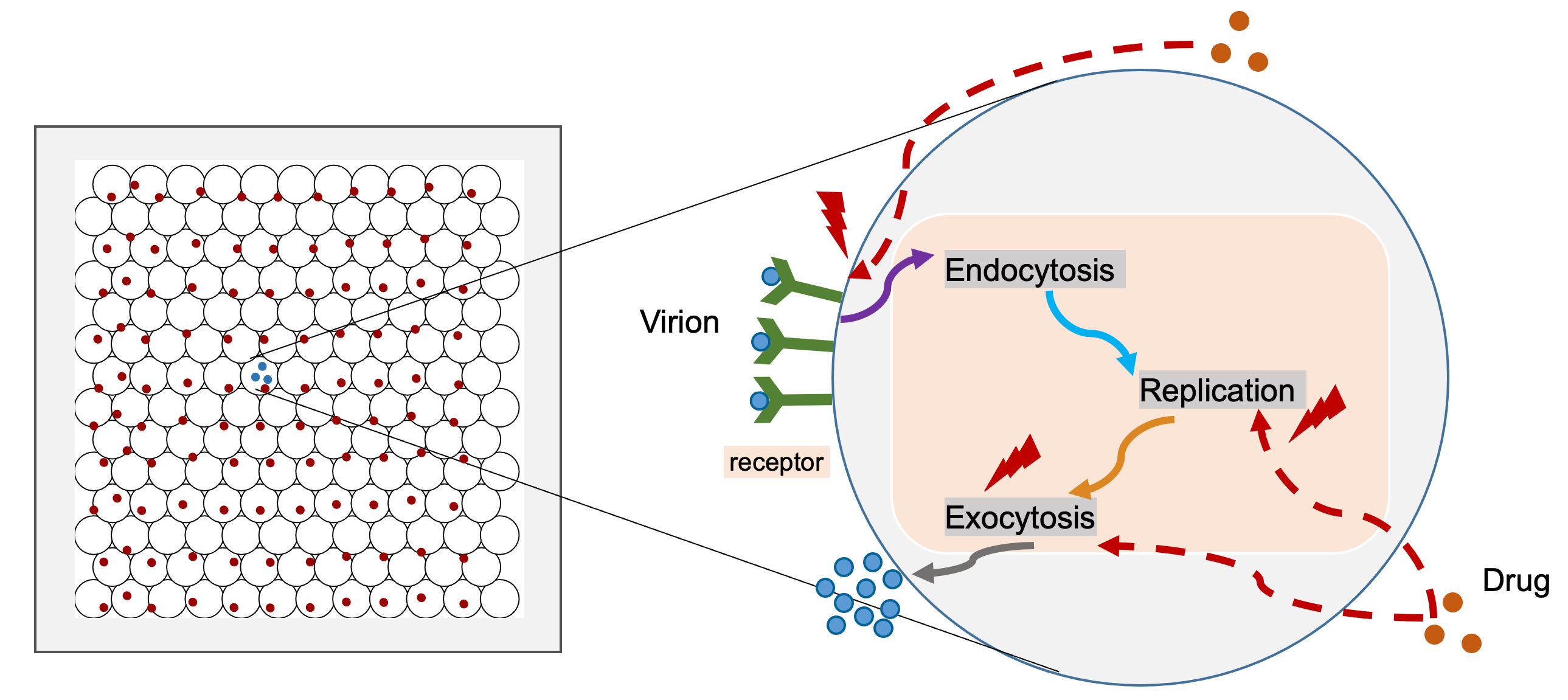
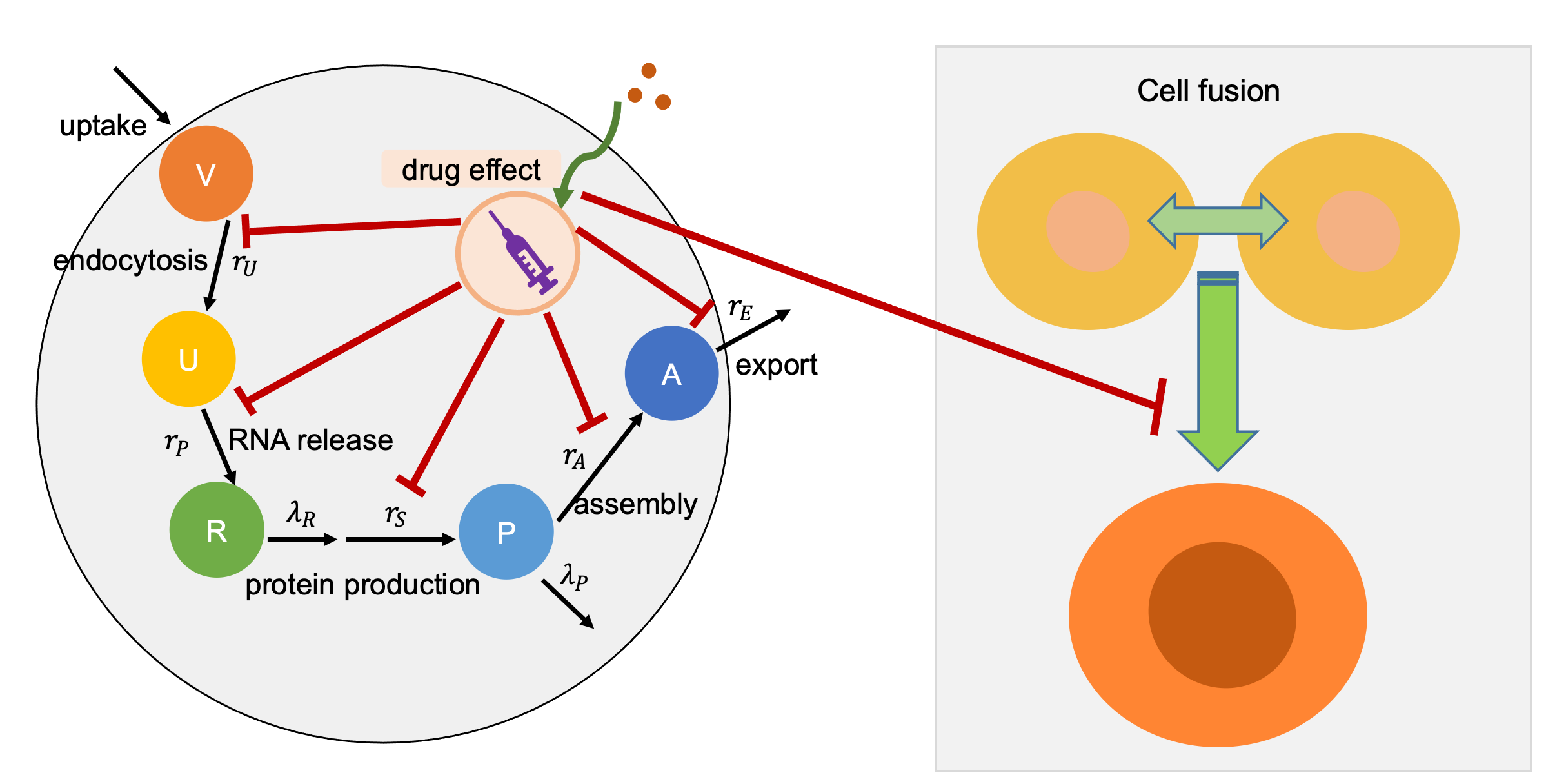
Please cite this project via the preprint:
Michael Getz et al., Iterative community-driven development of a SARS-CoV-2 tissue simulator. bioRxiv 2020.04.02.019075, 2020, (Preprint). doi: 10.1101/2020.04.02.019075.
Legend:
- lung epithelium: are colored by their (assembled) virion loads in four colors
- . Cells with 0 assembled virions.
- . Cells with 1-9 assembled virions.
- . Cells with 10-99 assembled virions.
- . Cells with 100-999 assembled virions.
- . Cells with 1000+ assembled virions.
- . Apoptotic (dead from viral load)
- Cell fusion: are colored by how many times of fusion
- . Cell fusion times within [1,2]
- . Cell fusion times within (2,4]
- . Cell fusion times within (4,6]
- . Cell fusion times within (6,8]
- . Cell fusion times within 8+
- Background: Contour plot of released virus that is diffusing in and above the tissue.
Caveats and disclaimers
This model is under active development using rapid prototyping:
- It has not been peer reviewed.
- It is intended to drive basic scientific research and public education at this stage.
- It cannot be used for public policy decisions.
- It cannot be used for individual medical decisions.
This model will be continually refined with input from the community, particularly experts in infectious diseases. The validation state will be updated as this progresses.
GUI Overview
- Config Basics tab: input parameters common to all models (e.g., domain grid, simulation time, choice/frequency of outputs)
- Microenvironment tab: microenvironment parameters that are model-specific
- User Params tab: user parameters that are model-specific
- Cell Types tab: parameters for cell types that are model-specific
- Out: Plots tab: output display of cells and substrates
- Animate tab: generate an animation of cells
Clicking the 'Run' button will use the specified parameters and start a simulation. When clicked, it creates an "Output" widget that can be clicked/expanded to reveal the progress of the simulation. When the simulation generates output files, they can be visualized in the "Out: Plots" tab. The "# cell frames" will be dynamically updated as those output files are generated by the running simulation. When the "Run" button is clicked, it toggles to a "Cancel" button that will terminate (not pause) the simulation.
Model details
This model is being rapidly prototyped. Biological and mathematical detail can be found at:
- Project website: http://covid19.physicell.org (opens in new tab)
- Model feedback: Google feedback form (opens in new tab)
- Preprint: Wang et al. (2020) (opens in new tab)
- GitHub codes: pc4covid19 GitHub organization (opens in new tab)
We request community help in estimating parameters and improving model assumptions at the link above.
This model and cloud-hosted demo are part of the education and outreach for the IU Engineered nanoBIO Node and the NCI-funded cancer systems biology grant U01CA232137. The models are built using PhysiCell: a C++ framework for multicellular systems biology.
Basic instructions
Modify parameters in the "Config Basics", "Microenvironment", "User Params", or "Cell Types" tabs. Click the "Run" button once you are ready.
To view the output results, click the "Out: Plots" tab, and move the slider bar to advance through simulation frames. Note that as the simulation runs, the "# cell frames" field will increase, so you can view more simulation frames.
If there are multiple substrates defined in the Microenvironment, you can select a different one from the drop-down widget in the Plots tab. You can also fix the colormap range of values.
Note that you can download full simulation data for further exploration in your tools of choice. And you can also generate an animation of the cells to play in the browser and, optionally, download as a video.
About the software:
This model and cloud-hosted demo are part of the education and outreach for the IU Engineered nanoBIO Node and the NCI-funded cancer systems biology grant U01CA232137. The models are built using PhysiCell: a C++ framework for multicellular systems biology [1] for the core simulation engine and xml2jupyter [2] to create the graphical user interface (GUI).
- A. Ghaffarizadeh, R. Heiland, S.H. Friedman, S.M. Mumenthaler, and P. Macklin. PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. PLoS Comput. Biol. 14(2):e1005991, 2018. DOI: 10.1371/journal.pcbi.1005991.
- R. Heiland, D. Mishler, T. Zhang, E. Bower, and P. Macklin. xml2jupyter: Mapping parameters between XML and Jupyter widgets. Journal of Open Source Software 4(39):1408, 2019. DOI: 10.21105/joss.01408.
Powered by
This software is powered by PhysiCell [1-2], a powerful simulation tool that combines multi-substrate diffusive transport and off-lattice cell models. PhysiCell is BSD-licensed, and available at:
- GitHub releases: https://github.com/MathCancer/PhysiCell/releases
- SourceForge downloads: https://sourceforge.net/projects/physicell/
It is a C++, cross-platform code with minimal software dependencies. It has been tested and deployed in Linux, BSD, OSX, Windows, and other environments, using the standard g++ compiler.
See http://PhysiCell.MathCancer.org.
The Jupyter-based GUI was auto-generated by xml2jupyter [3], a technique to create graphical user interfaces for command-line scientific applications.
References
[1] Ghaffarizadeh A, Heiland R, Friedman SH, Mumenthaler SM, Macklin P (2018) PhysiCell: An open source physics-based cell simulator for 3-D multicellular systems. PLoS Comput Biol 14(2): e1005991. https://dx.doi.org/10.1371/journal.pcbi.1005991
[2] Ghaffarizadeh A, Friedman SH, Macklin P (2016) BioFVM: an efficient, parallelized diffusive transport solver for 3-D biological simulations. Bioinformatics 32(8):1256-8. https://dx.doi.org/10.1093/bioinformatics/btv730
[3] Heiland R, Mishler D, Zhang T, Bower E, Macklin P (2019) Xml2jupyter: Mapping parameters between XML and Jupyter widgets. J Open Source Software 4(39):1408. https://dx.doi.org/10.21105/joss.01408
[4] Getz M et al. (2020) Iterative community-driven development of a SARS-CoV-2 tissue simulator. bioRxiv preprint 2020.04.02.019075. https://doi.org/10.1101/2020.04.02.019075
[5] Risner K et al. (2020) Maraviroc inhibits SARS-CoV-2 multiplication and s-protein mediated cell fusion in cell culture. bioRxiv preprint 2020.08.12.246389. https://doi.org/10.1101/2020.08.12.246389
Cite this work
Researchers should cite this work as follows:
-
Please cite:
Michael Getz et al. (2020), Iterative community-driven development of a SARS-CoV-2 tissue simulator. bioRxiv preprint 2020.04.02.019075. https://doi.org/10.1101/2020.04.02.019075